- Over the past few years, CBD-rich strains have proved to be real game changers in the cannabis market.
- The cannabinoid has gained popularity not only for its medicinal properties but also for its amazing recreational potential.
- Before placing a new strain on the market, we at Dinafem Seeds test it for cannabinoids and terpenes so that we can ensure both profiles meet our quality standards.
- We believe in the importance of research and attention to detail, and enjoy sharing the knowledge we have gained on the plant’s chemical composition. In this article, our lab techs explain how CBD is synthesised.
It is now widely known that Cannabis is a monotypic genus that consists of a single species: Cannabis sativa, as it was described by Leonard Fuchs in the 16th century (1). As a consequence of breeding and selection, a large variation of cultivars has been developed over the years, mostly for the recreational market, with famous names like Critical + 2.0, Original Amnesia, and Skunk.
Nowadays, more and more breeding programs are focusing on medical cannabis, and are therefore targeting different cannabinoid contents, mainly focusing on cannabidiol (CBD), but also on other cannabinoids like cannabidivarin (CBDV) or cannabigerol (CBG). Whether it is for recreational or medical purposes, these cultivars contain the same cannabinoids but in different proportions, which is why talking about fingerprints or chemovars, and classifying cannabis strains according to their cannabinoid content instead of their phenotype, would be more accurate.
Cannabinoid and terpenoid profiles are the characteristics that make one strain unique. These chemical compounds typically accumulate in considerable amounts in the secretory cavity of the glandular trichomes, mostly present in female flowers and in the aerial parts of the plant, and are responsible for the effects, taste, and scent of the plant (2). It has to be noted that terpenes can also be found in the roots of the plants, while trace amounts of cannabinoids can be found in seeds, roots and pollen.
In the last few years, various seed banks including Dinafem Seeds have engaged in breeding projects aimed at developing CBD-rich strains, as the increasingly in-vogue cannabinoid brings great value to medicinal and recreational users alike.
When was CBD discovered?
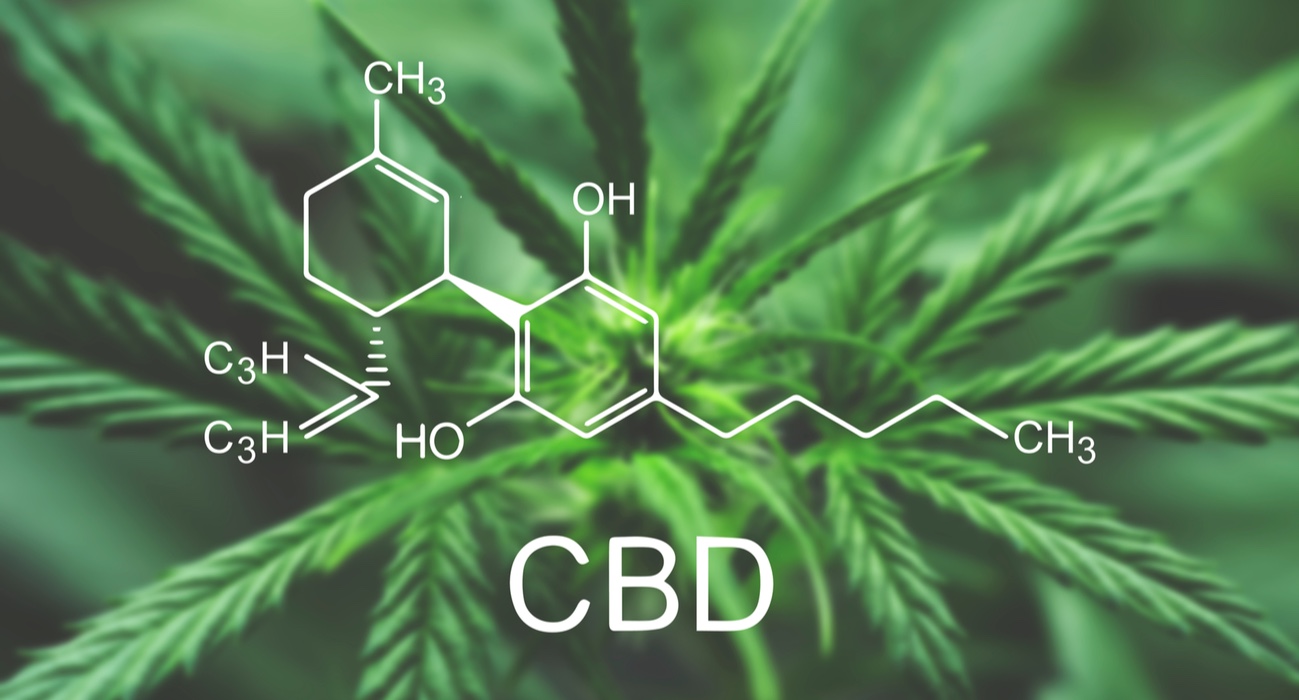
Cannabidiol was first isolated in 1940 (Adams), and then identified in 1963 (Mechoulam and Shvo) (3). Its chemical structure is similar to THC's; therefore, isomerisation can occur in harsh acidic conditions, i.e. CBD can be converted into THC by a simple chemical reaction. This is not likely to happen in humans, though, as gastric pH is not acidic enough to allow the reaction.
Today, more than 113 cannabinoids have been discovered and reported in the literature even though some of these are breakdown products only present in the plant in trace amounts (4). In this article we will focus on the synthesis of CBD.
How is CBD synthesised?
Over the years, the biosynthesis pathway leading to cannabinoid production has been elucidated, and it has been demonstrated that cannabinoids and terpenoids are chemically related as they share the same ancestor/precursor: Isopentenyl pyrophosphate (IPP), which comes from fatty acids (5). This structural similarity explains why beta caryophyllene (sesquiterpenoid) binds to CB2 receptor to produce its biological effects, and why fatty acid intake into the body can alter endocannabinoid levels.
CBD biosynthesis takes place in the trichomes (small hair or other outgrowth from the epidermis of a plant, typically unicellular and glandular) and is closely related to terpene biosynthesis, which is the reason why cannabinoids and terpenes can be considered as parent molecules. CBD biosynthesis involves olivetolic acid and geranyl pyrophosphate, and is mediated by an enzyme, CBDA synthase. This reaction leads to the production of CBDA, which acts as the precursor of CBD synthesis. This acidic form of CBD is mostly found in fresh cannabis samples. CBD is then obtained by the decarboxylation (removal of CO2) of its precursor CBDA. Decarboxylation can occur because of UV light exposure, prolonged storage or heat.

This mechanism explains why CBDA is mostly found in fresh cannabis samples, whereas CBD is produced when cannabis is smoked or dried. As explained, IPP can lead to both cannabinoids and terpenes. In order to produce cannabinoids and monoterpenes, IPP is first converted to Geranyl Pyrophopshate (GPP), whereas for producing sesquiterpenes it is converted to farnesyl pyrophosphate (FPP).
These precursors then react with different compounds and enzymes according to the biosynthesis pathway, leading to the formation of two main cannabinoids – the acidic compounds CBGVA and CBGA – which are then synthesised into other main acidic cannabinoids via different routes: CBDA synthase leads to CBDA, THCA synthase to THCA and CBCA synthase to CBCA. The genes responsible for the expression of these enzymes can express themselves in the plant to varying degrees, resulting in different cannabinoid productions, and are therefore responsible for the singularity of the strains' cannabinoid profiles.
This is where breeding and selection programs are focusing. Breeders cross plants showing the traits of interest in order to create new generations that express the desired gene and therefore produce the cannabinoid profiles fitting their requirements. In the end, when the flowers are properly dried and cured, without excessive light or heat, they mostly contain cannabinoids in the acidic form.
Because it is not an enzymatic reaction, decarboxylation of the cannabinoids (loss of the COOH group) is considered as degradation. While it can occur in the plant, the reaction is typically triggered when the flowers are smoked or baked into edibles, leading to the production of THC, CBD, CBC and CBG, the "active forms" of cannabinoids. Another degradation product is CBN, which is formed via THC, and is a clear sign of plant deterioration (6).
In addition to plant biosynthesis, and thanks to scientific advancements, cannabis plants are not the only source of cannabinoids. Microorganisms such as yeast and bacteria can be genetically modified to produce cannabinoids by inserting genes that code the appropriate enzymes to produce a desired compound (7). This can be of huge use in the medical market, as only a specific cannabinoid or cannabinoid group is produced. As a result, highly pure and reproducible pharmaceutical grade products can be obtained, which is a difficult task either by extraction from cannabis plants or by chemical synthesis.
The question is now whether we will be capable of scaling this up to the point where microorganism produced cannabinoids is worth the effort. Basically, making a lot of it is the bigger challenge right now.
- Hazekamp. Chemistry of Cannabis. Comprehensive Natural Products II. (Eds: L.Mander, H-W. Lui). Elsevier, Oxford, 2010, 3, pp. 1033-1084.
- Hazekamp; J.T. Fichedick. Cannabis – from cultivar to chemovar. Drug. Test. Anal. 2012, 4, 660-667.
- G. Pertwee. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 2006, 147, S163-S171.
- Aizpurua-Olaizola, et al. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J Nat Prod. 2016, 79, 324-331.
- Russo. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011, 163, 1344–1364.
- Aizpurua-Olaizola, et al. Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2014, 406, 7549-7560.
- L. Poulos; A.N. Farnia. Production of cannabinoids in yeast. US20160010126A1 patent, 2017.



Comments from our readers
There are no comments yet. Would you like to be the first?
Leave a comment!Did you like this post?
Your opinion about our seeds is very important to us and can help other users a lot (your email address won't be made public).